Guidance and Support for Enhanced Biosecurity
Below we have provided recommendations for enhanced biosecurity when working with your amphibian collection. These actions minimize potential disease spread in your amphibians as well as reduce risk of pathogens reaching wild amphibian populations.
Remember it's always possible that individuals you have are harboring pathogens on their skin even if they don’t look sick. Biosecurity is important even when you don’t see visual signs of poor health.
We are here to help if you have questions. We are happy to arrange a zoom or phone call to you to discuss these recommendations (Healthy Trade Institute - info@healthytrade.org, phone: 617-505-8165 Molly Bletz or 865-385-0772 Matt Gray).
Enhanced Biosecurity Protocols
Use of Gloves
Purpose: Using disposable gloves can prevent the movement of pathogens between groups of animals, ensuring those involved in husbandry do not become inadvertent “vectors” of infection. There is plenty of evidence that contaminated gloves can result in transmission of amphibian pathogens from an infected to uninfected individual (see Gray et al. 2018).
Procedure:
-
Disposable gloves should be worn while handling, feeding, or otherwise caring for animals and changed between containers.
-
Gloves can contain powders or other unknown chemicals on them that may be harmful to animals, so it is recommended that gloves first be rinsed with fresh water before handing animals (see Greer et al. 2009).
-
Since it can be difficult to get gloves on wet or sweaty hands, one can “double glove,” putting on one set of gloves on top of another, and simply change the outer layer.
-
Ideally, gloves should be changed between housing containers (independent habitats) unless there is strong confidence that animals are pathogen-free (Gray et al. 2018).
Use of Disinfectants at Approved Concentrations
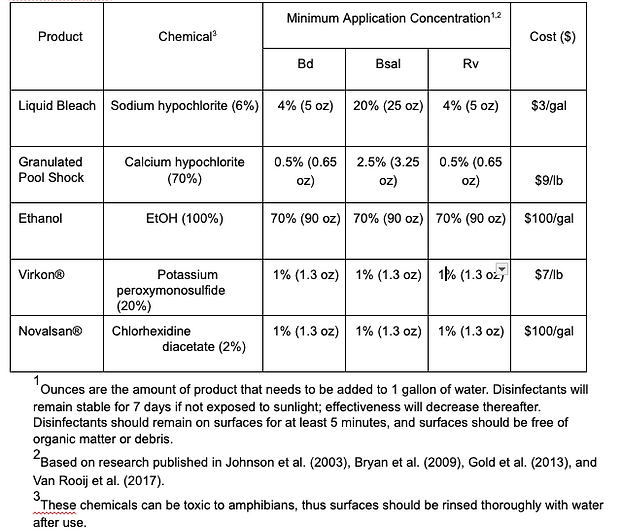
Purpose: Disinfectants are used to inactivate pathogens and may be used prophylactically (=preemptively) or in response to known contamination. There are many types and brands of disinfectants that can be organized by their active ingredient. They may have different efficacies against various pathogens, different working concentrations and required contact times, different longevities (and thus frequency of being remixed), and different costs and benefits (see Table 3)..
Table 3. Common disinfectants used for inactivating Batrachochytrium dendrobatidis (Bd), B. salamandrivorans (Bsal), and ranavirus and approximate cost (in 2023 USD).
Note on containment and labeling:
-
It is best practice to place disinfectants in a secondary container (e.g., a bin or bucket) in case of spills. Be sure to physically separate caustic chemicals from other chemicals or supplies.
-
Be sure containers are clearly labeled with the contents and the date of purchase and expiration date. Containers with diluted chemicals should be similarly labeled with the contents and date when mixed up.
Disinfecting surfaces
-
Use a spray bottle or apply to a clean rag or paper towel to apply enough of the appropriately diluted disinfectant to the surface that it is wet and the entire surface is covered.
-
Let sit for the required contact time (see Table 3). A timer is helpful.
-
Wipe up with a clean rag or paper towel.
-
If necessary (e.g., if the disinfectant is corrosive such as bleach or the surface will contact animals) rinse several times with clean water to remove any residue.
Disinfecting equipment
-
Fill a tub with appropriately diluted disinfectant and soak equipment for required contact time, then rinse thoroughly and dry.
-
Note that some chemical disinfectants are inactivated by organic matter and should be disposed of after disinfecting many dirty items.
-
Alternatively, spray the equipment with appropriately diluted disinfectant being sure to cover all surfaces thoroughly, letting it sit for the required contact time before rinsing thoroughly.
Disinfecting water or spills
-
Wastewater should be collected (e.g., in a bucket) and then the appropriate disinfectant (e.g., bleach) can be added to the water to achieve the required dilution and mix thoroughly to ensure a homogenous solution. Let sit for the required contact time before disposing of water as per usual.
-
Note that because some chemical disinfectants are inactivated by organic matter, etc., it may be appropriate to aim for a higher concentration than that recommended (e.g., 10% bleach instead of 5% bleach). Granulated chlorine (used for shocking pools) can be an effective way to disinfect large volumes of water.
-
Small spills can be sprayed with disinfectant, wiped up with a disposable rag or paper towel, sprayed again and let sit for the required contact time before being wiped up again and the area rinsed.
-
Large spills may require adding a higher concentration of the disinfectant so that the overall concentration is at least that recommended for disinfection before being mopped up. The area should then be sprayed again with disinfectant, let set for the required contact time, and then wiped up and rinsed.
Quarantining and Isolating Animals
Purpose:
Quarantine is intended for newly arrived animals to ensure they are healthy before they are allowed to mix with and potentially infect resident animals.
Isolation is intended for animals that have become sick, tested positive for infections, or were co-housed or exposed to sick or infected animals. The goal is to contain (potentially) affected animals to prevent further transmission while responding to the infections.
Housing and Infrastructure
-
Ideally there will be a physically separate room for quarantine and isolation. At a minimum, a distinct, physically separated area of an animal room could be reserved for quarantine and isolation.
-
Ideally both the walls and floor of the room or area should be easily disinfectable (i.e., have non-porous surfaces, minimal cracking, chemically-resistant paints).
-
Ventilation is important, especially when chemical disinfectants are used regularly, but ventilation of the quarantine room should ideally be separated from that of the non-quarantine room(s).
-
Separate housing containers (i.e., tanks, tubs, containers), equipment (e.g., nets, forceps, funnels & tubing, etc.), water and food should be used in quarantine and isolation spaces. That is, there should be no mixing of anything that might incidentally carry or contain pathogens (= “fomites” in the literature).
-
Waste of all kinds—water, bedding, trashing—should be kept physically separate from that of other rooms and must be appropriately treated before being disposed of (see Disposal SOP later in the Plan).
-
To prevent the movement of pathogens out (or into) the quarantine room or area, there should be separate footwear (e.g., sandals or boots) and coats or outerwear at the entrance that are donned and used only in the quarantine room or area. At a minimum, the entry should include a disinfectant footbath that is regularly filled and cleaned.
Order of operations
Quarantine and isolation areas or rooms should be entered last, after working with the rest of the animals. Ideally, personnel that enter the quarantine will not re-enter other animal areas until after changing clothes or otherwise ensuring no pathogens can re-enter with them.
Biohazardous Disposal Options of Dead Infected Animals
Purpose: Animal carcasses can harbor many viable pathogens and so should be disposed of to ensure no further transmission or contamination is possible. Infected animals may also be of great interest and utility to researchers (e.g., for pathology, pathogen isolation, DNA analyses). We recommend that a subsample of diseased animals (up to 20) is sent to the researchers (Table 1), while the remaining animals can be decontaminated then disposed.
Animals for Researchers
If animal carcasses are destined for researchers, ideally the researchers should be contacted to provide preferred options for storing or preserving animals. In general, we recommend that half of the animals are kept cold (but not frozen) and the other half are frozen. Another option is to preserve the animals in 70% ethanol. Details are provided below.
-
Amphibians are kept cold in the refrigerator, but not frozen, for histological examination or pathogen isolation. Carcasses should be placed in individual bags (e.g., Ziploc bags) that are then placed in a secondary bag or container to prevent leaks. Animals should be kept separate of food or supplies, ideally in a separate refrigerator.
-
Preserved frozen, for pathogen isolation generally placed in individual bags that are then placed in a secondary bag or container to prevent accidental leaks.
-
Labels written on pieces of paper are preferable to using pen or marker on the bags, which may come off with chemicals or in ultracold freezers.
-
A typical frost-free freezer will occasionally warm up to defrost before cooling down again. This freezing and thawing tends to degrade samples, so if non-frost-free freezer is available, this would be preferable. If not, the samples can be surrounded by ice blocks or the equivalent.
-
Ethanol preserved, usually with ≥70% ethanol (as opposed to “rubbing alcohol”,
-
isopropanol).
-
Glass (Mason) jars are best as ethanol will leak through or degrade many plastic containers. Glass jars should be wrapped in bubble wrap or a similar material to prevent jostling.
-
In general, there should be ≥ 10 times as much ethanol as volume of animals.
-
Larger animals can be cut open to allow the ethanol to penetrate internal tissues for better preservation.
Animals for Immediate disposal
There are several options for disposal depending on what options are locally available:
-
Small and soft-bodied animals can be placed in a high concentration of commercial bleach (e.g. 50% bleach) for at least 6 hours to inactivate most pathogens. Use of disposable gloves and splash goggles are recommended when handling bleach.
-
Animals can be autoclaved, if available, or baked at 250°F (120°C) for 30-60 minutes before disposal.
-
Decontaminated animals should be placed in double-layered, opaque trash bag, and closed using a square-knot. The outside of the bag should be disinfected prior to depositing in regular trash.
-
Burial or disposal outside should be avoided as the carcasses may be dug up and can contain large concentrations of viable pathogens.
-
A final option is to contact a biohazardous waste company, such as Stericycle, and arrange for pick up. Stericycle will have biohazardous bags for waste. Amphibians can be put in a regular, double layered trash bag and frozen prior to pick up.
General notes
-
Gloves should be worn when handling animals and changed before touching any other surfaces or materials.
-
The outside of the secondary bag or container should be sprayed with an appropriate disinfectant to inactivate any pathogens that might have contaminated the outside of the bag or container.
Proper Disposal of Potentially Contaminated Aquarium Contents
Purpose: Materials in aquaria or terraria that have housed (potentially) infected animals can harbor pathogens and thus serve as a source of infection to other animals. Proper disposal ensures the risk of further transmission is minimal.
Procedure:
-
Substrates, plants, wood, and other structures should be soaked in an appropriate disinfectant for the required contact time before being disposed of in the trash.
-
Note that organic material can inactivate certain disinfectants (e.g., the active ingredient in bleach) and so should be mixed at a higher concentration when disinfecting aquarium contents.
-
Autoclaving aquaria and their contents is also a possibility.
Disinfecting water
-
Wastewater should be collected (e.g., in a bucket) and then the appropriate disinfectant (e.g., bleach) can be added to the water to achieve the required dilution and mix thoroughly to ensure a homogenous solution. Let sit for the required contact time before disposing of water as per usual.
-
Note that because some chemical disinfectants are inactivated by organic matter, etc., it may be appropriate to aim for a higher concentration than that recommended (e.g., 10% bleach instead of 5% bleach). Granulated chlorine (used for shocking pools) can be an effective way to disinfect large volumes of water.